Intra-alveolar injury and injury following systemic inflammation of the lungs are the factors which cause acute lung injury which, in its most severe form, results in acute respiratory distress syndrome. This syndrome is a common cause of mortality in hospitals and is currently only managed by treating its underlying causes (for example severe respiratory infection), haemodynamic support, and mechanical ventilation. A new and exciting potential treatment for this syndrome, and many others like it, is being developed in stem cell research. If successful, stem cell manipulation can promote regrowth and healing of damaged lung parenchyma. Research is slow however, as it is hindered by the fact that the mature lung is formed from at least 40 different stem cell lineages, of which each is morphologically differentiated.
Stem cells are cells with a limitless capacity for self-renewal and reinvigorate themselves via mitotic cell division and have the ability to differentiate into any number of cell types. They are known for their plasticity, meaning their ability of the cells to cross cell lineage boundaries. Simply put, a bone marrow stem cell, for example, has the ability to become any type of tissue in the body, from blood to lung tissue. Stem cells are accommodated in niches, or subsets of cells and extra-cellular components that can accommodate stem cells indefinitely and can control their proliferation and renewal.
 Figure 1 from Yen et al. (2006) showing the principle of stem cell plasticity.
Figure 1 from Yen et al. (2006) showing the principle of stem cell plasticity.Originally the alveolar type 2 (AT2) cells were thought to be the only stem cells of the lung parenchyma. AT2 cells compose 5% of the surface of the alveoli and are small, polarized epithelial cells which are cuboidal in shape. They have microvilli on the apical surface and organelles typical for eukaryotic cells. They are morphologically distinguished from alveolar type 1 (AT1) cells by the presence of lamellar bodies in the cytoplasm, which secrete the precursors for lung surfactant. Lung surfactants are phospholipids responsible for regulating alveolar surface tension. AT2 have the unique ability to transport sodium from the alveolar space. Water and chloride naturally follow this gradient so the AT2 cells are able to clear fluid from the lungs, which is important in resolving pulmonary edema and inflammatory lung injury.
The original model for stem cell differentiation in the lung parenchyma involved the proliferation and division of the AT2 cells. The AT2 cell was said to give rise to two daughter cells—one of which differentiated into an AT1 cell and the other which remained an AT2 cell. This would typically be stimulated by the loss of either AT1 or AT2 cells. Yen et al. (2006) have brought attention to a significantly modified stem cell model which fits much better with the current knowledge and research on stem cell proliferation. They propose that the AT2 cells maintain in the G0 stage of cell division, meaning they are quiescent. When stimulated by the loss of a nearby AT1 or AT2 cells, they re-enter the cell cycle and divide into two undifferentiated daughter cells which have the potential to become AT1 or AT2 cells. Both AT1 and AT2 cells are capable of cell mediated death (apoptosis) in order to maintain the balance of the epithelial cell population. Exogenous stem cells which are derived from bone marrow and are involved both in later stages of normal lung repair and in repair of serious injury are capable of differentiating into AT1 or AT2 cells depending on the epithelial requirement.
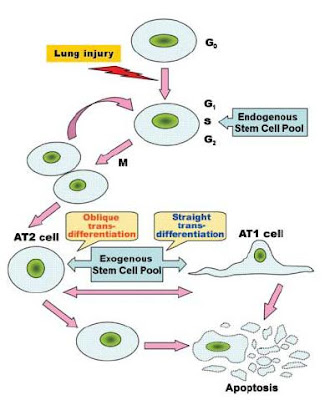
Figure 2 from Yen et al. (2006) showing the resources of stem cells of the alveolar epithelium from endogenous and exogenous pathways.
As significant research on stem cells has only been pursued since the 1960s, few molecular signals which regulate stem cells of the lung parenchyma have been discovered. The growth factors KGF and HGF promote the growth, division, and migration of AT2 cells. Retinoic acid (a derivative of vitamin A) has been shown to stimulate the proliferation of AT2 cells as well, including the stimulation of lung repair proceeding injury. It is thought that these effects of retinoic acid are caused by the molecules affecting the genes responsible for lung morphogenesis and increasing recruitment of bone-marrow derived stem cells into the lung.
Stem cell ability to regenerate and repair lung tissue looks to be a promising therapy for injury and disease. Pharmacological therapy could involve using novel growth factors and cytokines discovered to influence lung stem cells to stimulate the body to repair and regenerate itself. In fact, animal experimentation has revealed HGF, KGF, and retinoic acid injections stimulated AT1 and AT2 cell proliferation in the lung. Cellular therapy involves restoring function of damaged tissues via the transplantation of healthy cells. Stem cells have the potential to be transplanted to help restore function to damaged or diseased tissues. Mouse studies already indicate that embryonic stem cells can be used to generate cells which secrete insulin and other hormones, and other studies indicate stem cell transplantation can be used for lung repair. When autologous, stem cell transplantation would overcome the problem of immune rejection. Gene therapy includes the introduction of exogenous genetic material to correct or modify the function of a certain cell. Stem cells may resolve an important problem in gene therapy—the fact that high levels of gene expression without repeated gene transduction cannot be maintained. Genetic modification of stem cells would produce a population of genetically altered cells which would not require repeated procedures.

Figure 3 from Yen et al. (2006) showing prospects for stem cell therapies.
Although significant advances in stem cell research have been made, much more research is required before stem cell therapies become a useful and effective treatment for disease and damage of the lung parenchyma.
This article was exceptionally well written and extensively researched. It is a comprehensive review of relevant information and research on stem cells which can be applied to lung parenchyma injury therapy. Normal lung tissue is described as well as the characteristics of stem cells so the reader can better understand the full implications that stem cells will eventually have on modern medicine—especially disease therapy. The authors introduced a novel and exceptionally in depth model for alveolar epithelium kinetics and induction and proliferation of stem cells in lung (or any) tissue. Unfortunately, I found that the section on stem cell niches and plasticity were far too detailed for a review article, especially since the presence of niches is theoretical in many tissues still. These sections could have been half as long and still detailed enough to fit the flow of the article. Although short, the various therapeutic applications for stem cells the authors describe include both easy to understand explanations and several recent supporting experiments for each therapy. A weakness of this approach, however, is the simple fact that stem cell research is still very basic and the therapies the authors reference are at least a decade away from approaching clinical trials. A problem with the gene therapy section in particular is that it is very general, and only vaguely references lung repair as an application.
Overall this article was easy to read and organized exceptionally well. It is an excellent reference for any scientists or students who wish to enlighten themselves about the potential of stem cells on lung parenchyma repair and regeneration.